How does Venus prioritize products in its research pipeline, and what is the development process for bringing these products to market?
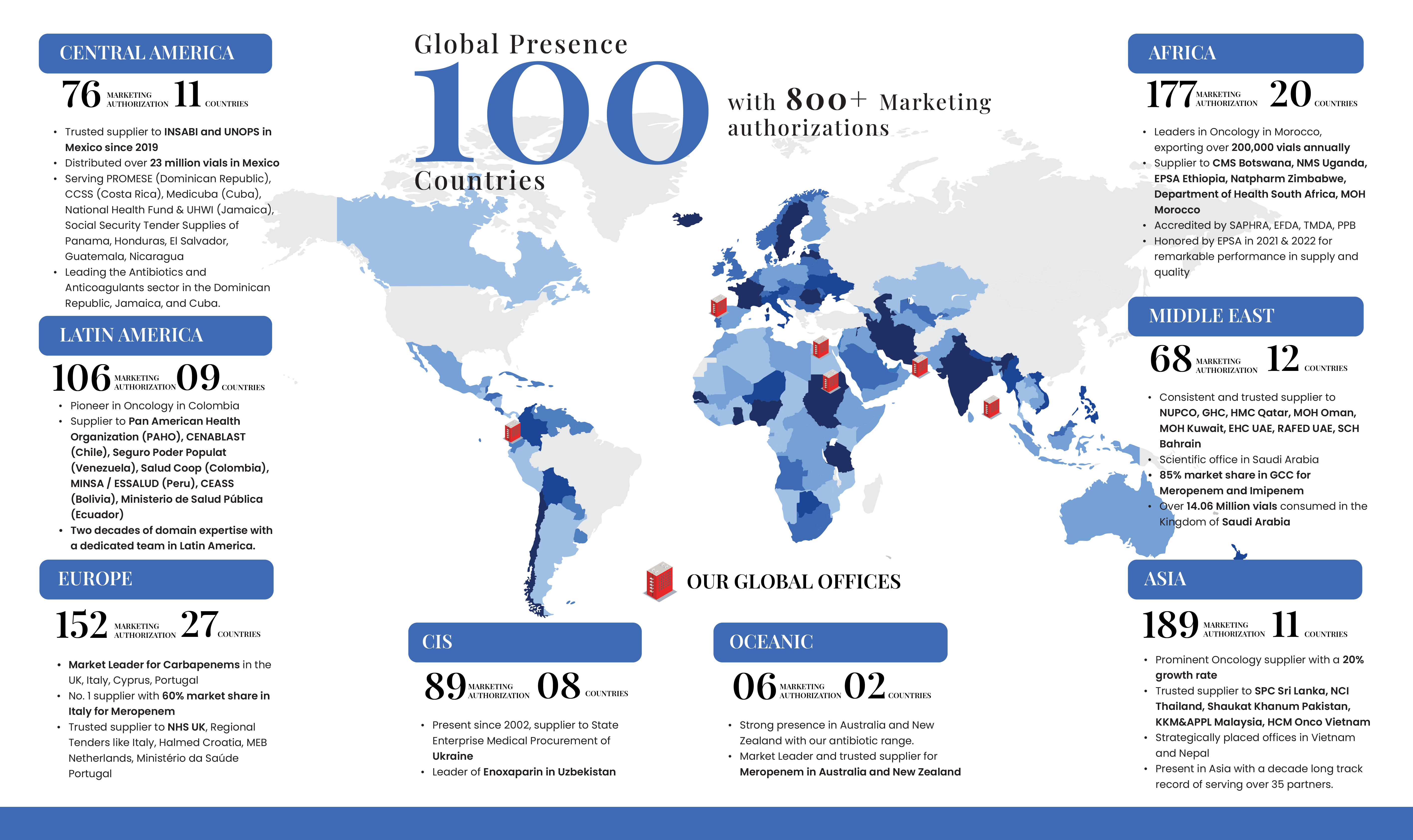
Venus follows a strategic approach in prioritizing products for its research pipeline, focusing on therapeutic need, innovation potential, and alignment with our long-term goals. Products that address critical health challenges and show promise for significant therapeutic impact are given priority. We actively pursue novel formulations and technologies that have the potential to make a meaningful difference in healthcare.
Currently, our pipeline includes several exciting products across various categories. In the Anti-Infective segment, we are developing VRP-034, VRP-044, and VRP-035, while our Natural Products pipeline includes VRP-019, VRP-1016, and VRP-1020. The Hemostatic category features VRP-029, VRP-030, and VRP-031. These products are in different stages of development, from pre-clinical research to advanced phases.
Once a product is prioritized, it undergoes a thorough and rigorous development process. This starts with detailed formulation research and pre-clinical studies to ensure both safety and efficacy. Following this, the product moves through multiple phases of clinical trials to further validate its effectiveness and ensure it meets the highest standards of quality and safety. Each step is meticulously planned to ensure that the product is well-researched and ready for market launch, ultimately delivering impactful solutions to patients and healthcare providers.
After the development phase, the Supply Chain department plays a critical role in managing the storage of pharmaceutical products. Products are stored in state-of-the-art, temperature-controlled warehouses designed to meet stringent regulatory requirements. These warehouses are also certified by the government and hold accreditations such as the three-star export house by the Government of India and the CII Responsible Export Organization certification. This reflects our dedication to ethical practices and high standards in our operations, including maintaining specific temperature and humidity levels, ensuring protection from light exposure and contamination, and adhering to all safety and hygiene standards necessary for pharmaceutical storage.
When the time comes for distribution, products are contained in controlled environments to preserve their integrity and effectiveness. This meticulous approach ensures that all pharmaceutical products maintain their quality during transit, reaffirming our commitment to delivering safe and effective solutions to healthcare providers and patients.
Our commitment to excellence is further demonstrated by our adherence to Good Distribution Practices (GDP), ensuring products are handled with the utmost care throughout their journey.
By carefully managing the prioritization and development process, Venus ensures that only the most innovative and high-potential products move forward, maximizing both therapeutic benefit and overall impact on healthcare.
Read more about us on our Social Media Handles and The Venus Times Newsletter.